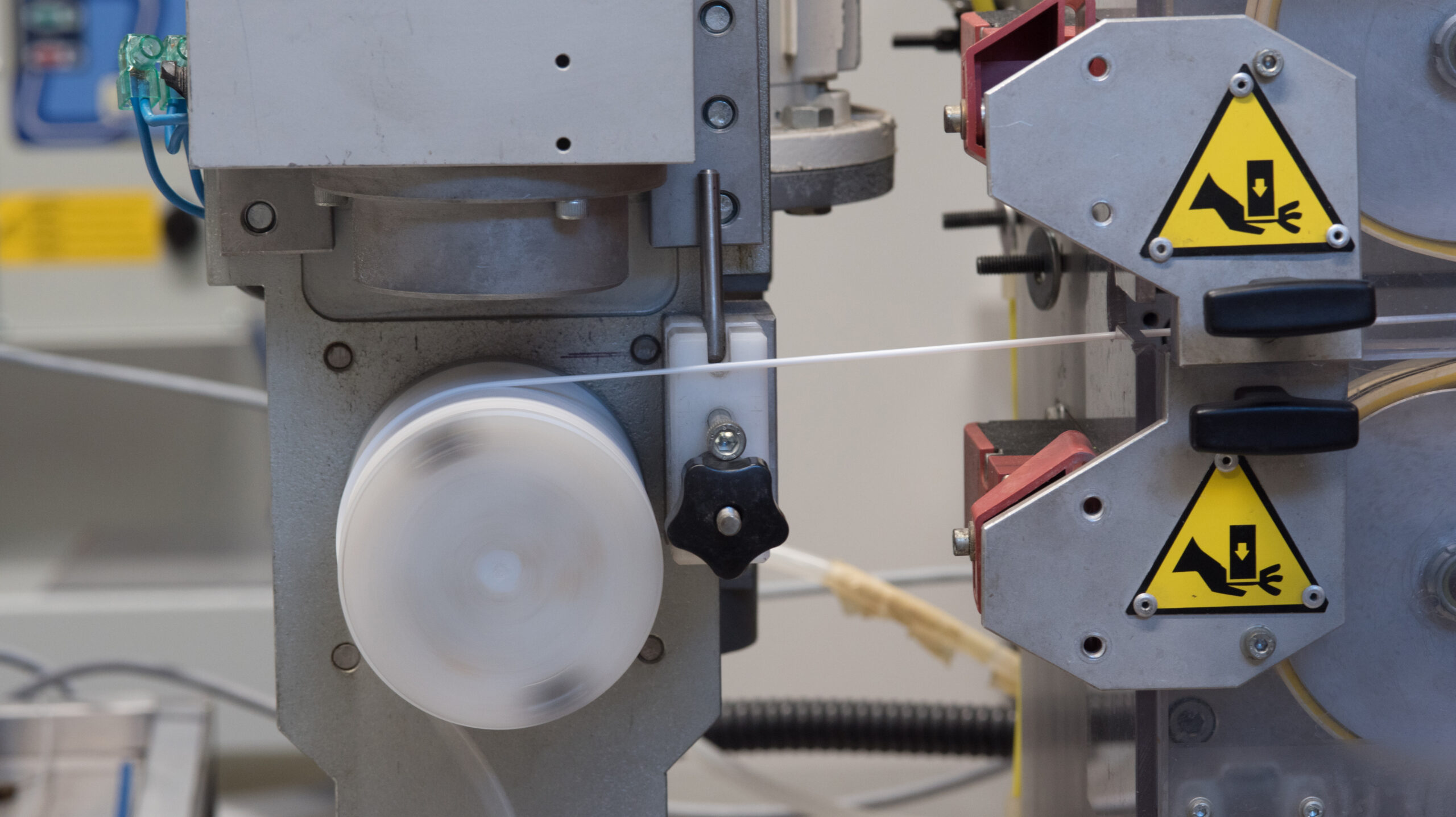
Controlled Extrusion Technology
Extrusion is a critical process that requires meticulous control of every parameter. MDE uses automated lines equipped with in-line laser control systems to constantly measure the diameter, wall thickness, and lumen concentricity, ensuring maximum micrometric precision and a repeatable, validated process.
Tubing Types and Materials
We develop tubes and micro-tubes in a wide range of medical-grade polymers depending on the clinical application (catheters, stent delivery, vascular access, etc.).
Main processing types:
Certified materials
Materials Medical-Grade Traceable
We exclusively use certified materials, ensuring full compliance with the ISO 10993 (biocompatibility) standard. Our R&D division can develop custom polymeric compounding for specific needs.
Main materials treated:
Extrusion Process in Cleanroom
All extrusion activities take place in ISO 8 Cleanrooms with controlled pressure, and constant monitoring of environmental parameters. This guarantees process purity and the elimination of particulate contamination risks, which is essential for medical devices.
Documentation
Quality Control and Validation
Every batch of extruded tubing undergoes rigorous metrological and functional checks. Tests include dimensional verification via optical microscopy and rheological analysis of materials. MDE provides complete IQ/OQ/PQ validation documentation.
FAQ
Diameters range from 0.2 to 10 mm, with tolerances of a few hundredths of a millimeter.
Single-layer and multilayer tubing in medical-grade materials for catheters, balloons, and infusion systems.
Yes, MDE develops custom formulations and co-extrudes materials with different properties.
Yes, each batch is accompanied by complete inspection reports and traceability data.
Yes, all extrusion lines operate in ISO 8-certified cleanrooms.