ISO 13485 Certification
ISO 13485:2021 is the international reference standard for quality management systems applied to the design, development, production, and assembly of medical devices. MDE guarantees the rigorous application of these standards across all its divisions, ensuring maximum reliability and traceability.
Compliance with the MDR Regulation
The Medical Device Regulation (MDR 2017/745) defines the legal and clinical requirements for access to the European market. Thanks to our integrated quality system, we can support customers in creating the complete Technical File and managing all compliance requirements.
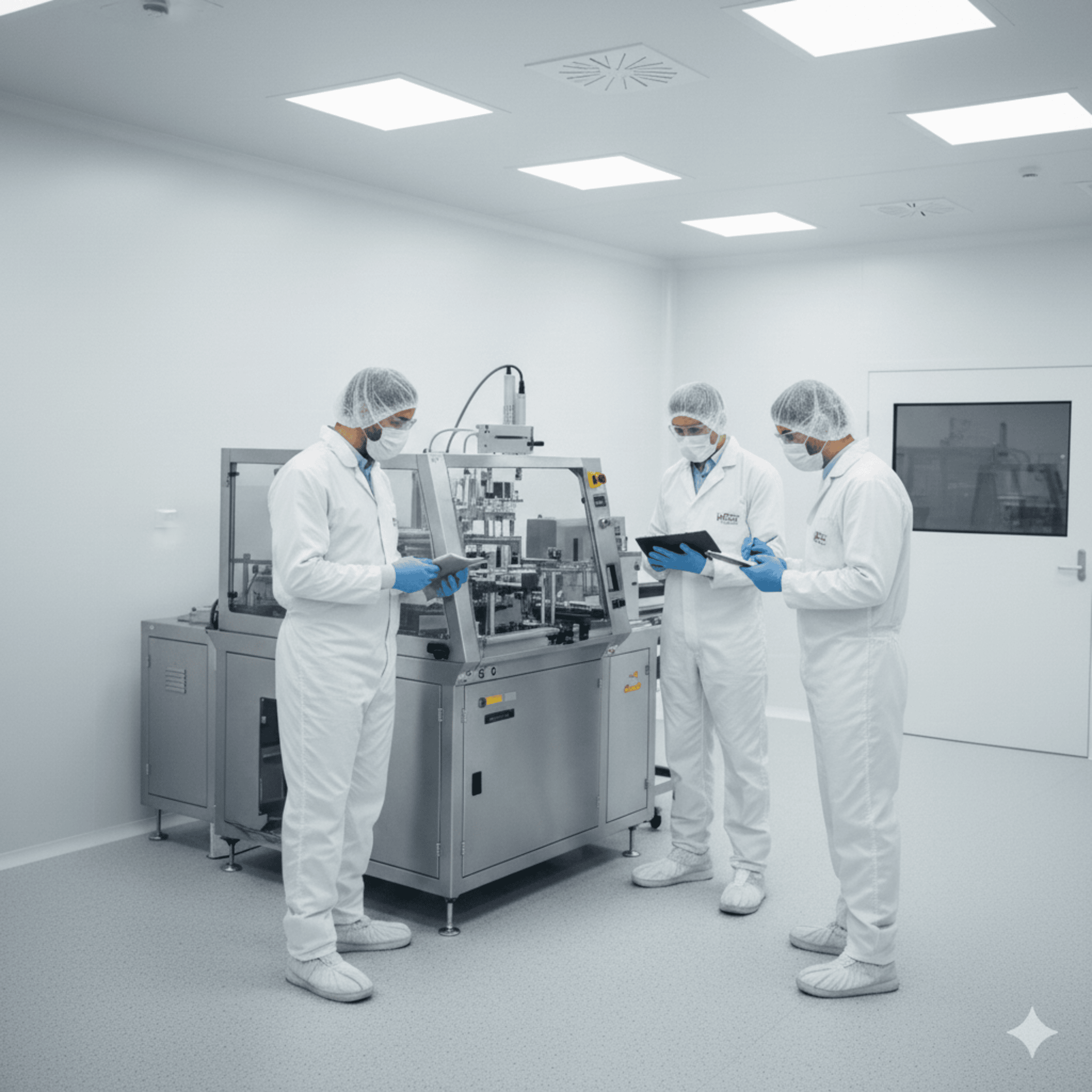
Production in the cleanroom.
Process Validation IQ/OQ/PQ
Validation is a crucial step in ensuring that the manufacturing process consistently produces devices that meet specifications. We use the IQ/OQ/PQ (Installation, Operational, Performance Qualification) methodology to certify machinery and production lines, particularly for cleanroom processes.
Traceability and Digitalization of Processes
MDE has implemented an integrated data management system that connects every machine, sensor, and production line to the central database. This allows for automatic association of:
ISO 14644
Clean Room
ISO 7 and ISO 8
Our ISO 7 and ISO 8 cleanrooms provide contamination-controlled environments, compliant with ISO 14644 standards. They are equipped with a high-performance HVAC system, which ensures:
FAQ
Yes, all processes and technical documentation comply with the European Medical Device Regulation.
MDE is UNI EN ISO 13485:2016 certified for the design and production of medical devices.
Each batch is accompanied by a complete technical dossier and digital records documenting all production phases.
Yes, MDE provides technical documentation and validation certificates for each supply upon request.
Each batch is accompanied by a complete technical dossier and digital records documenting all production phases.